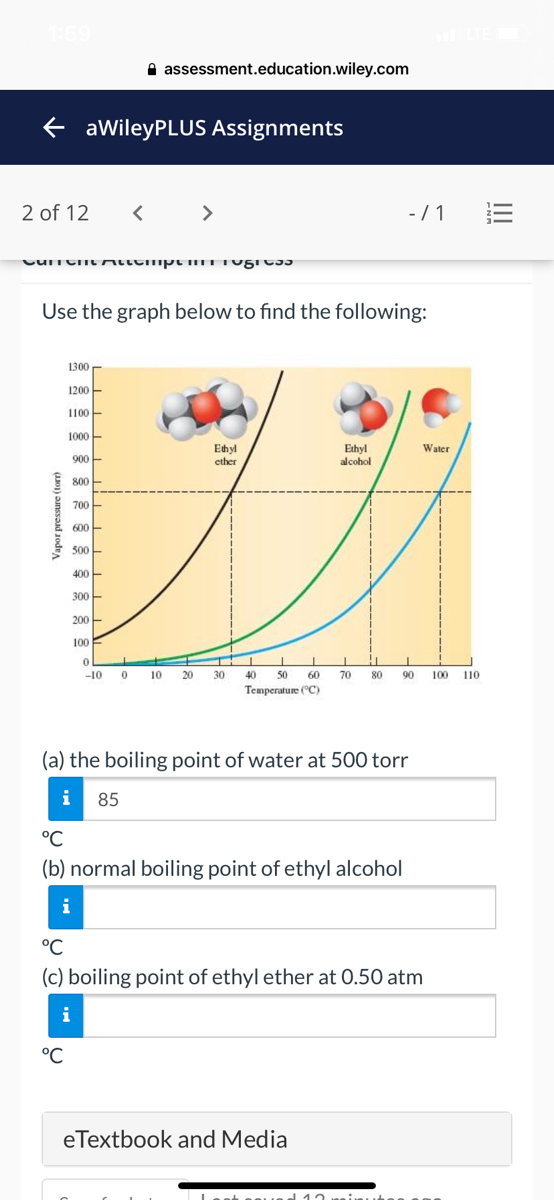
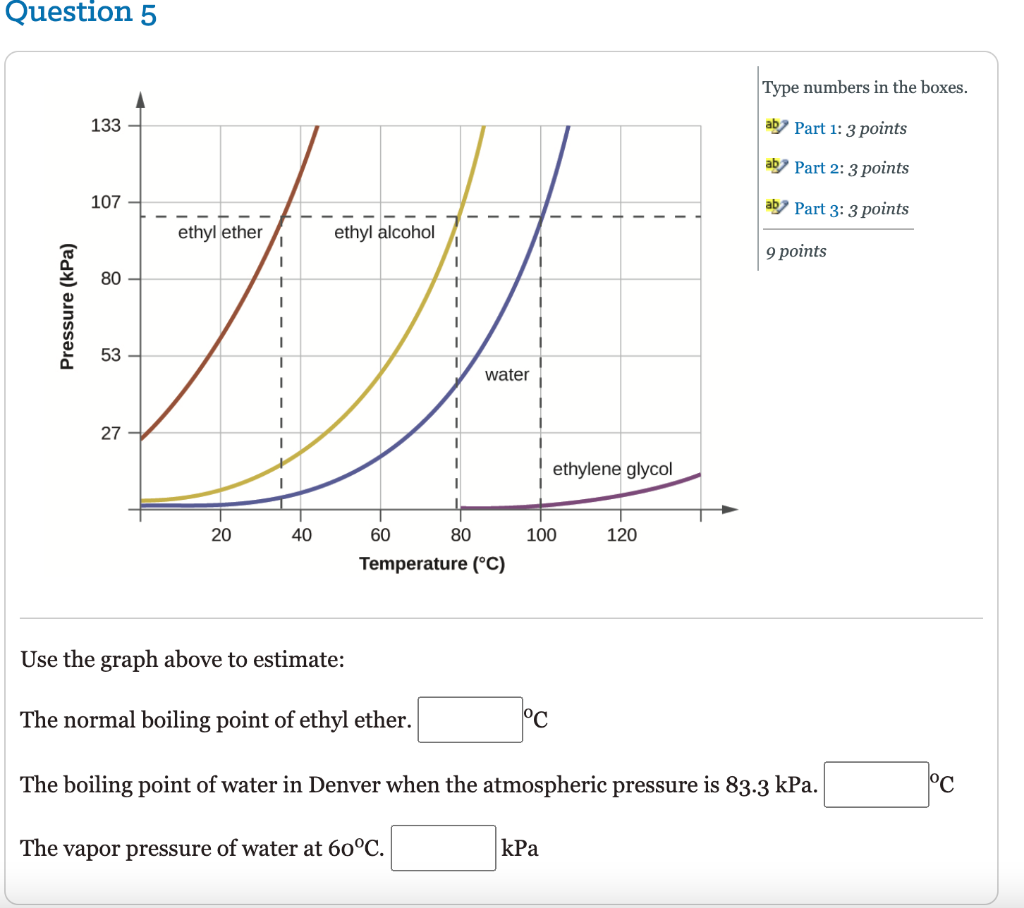
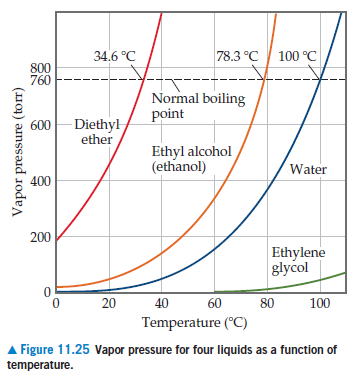
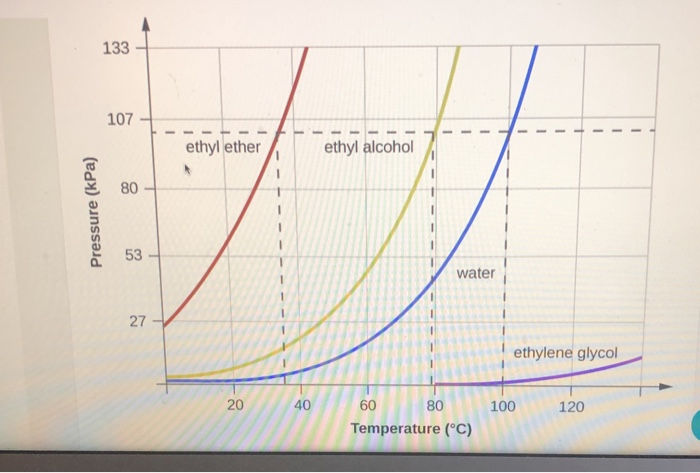
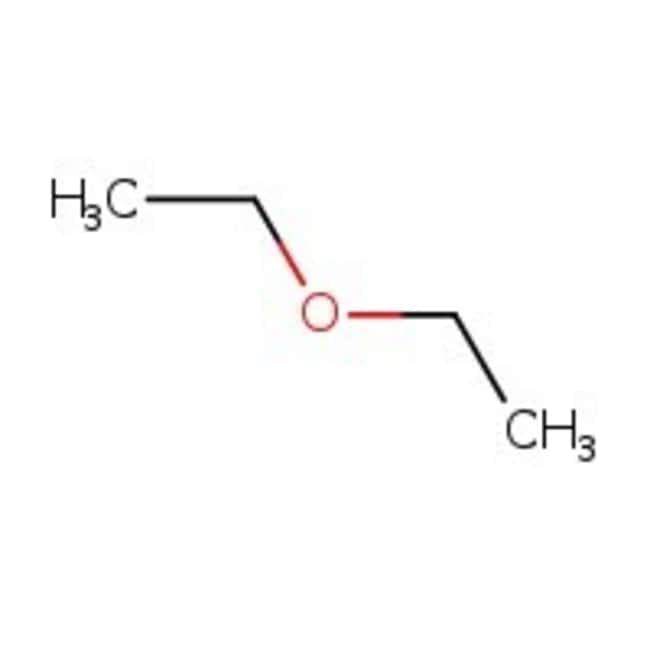
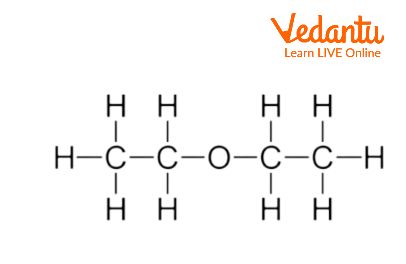
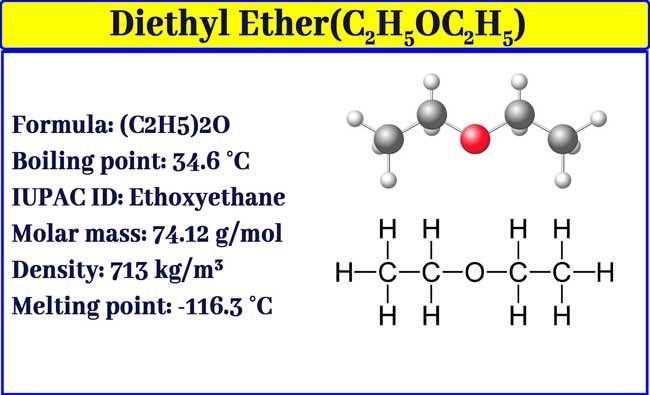
Diethyl ether has a normal boiling point of `35.0^(@)C` and has an entropy of vaporization of `84.4 - YouTube

cyclohexyl ethyl ether - C8H16O, density, melting point, boiling point, structural formula, synthesis
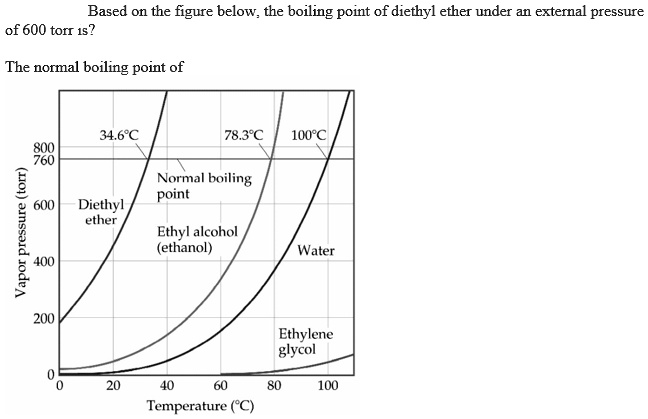
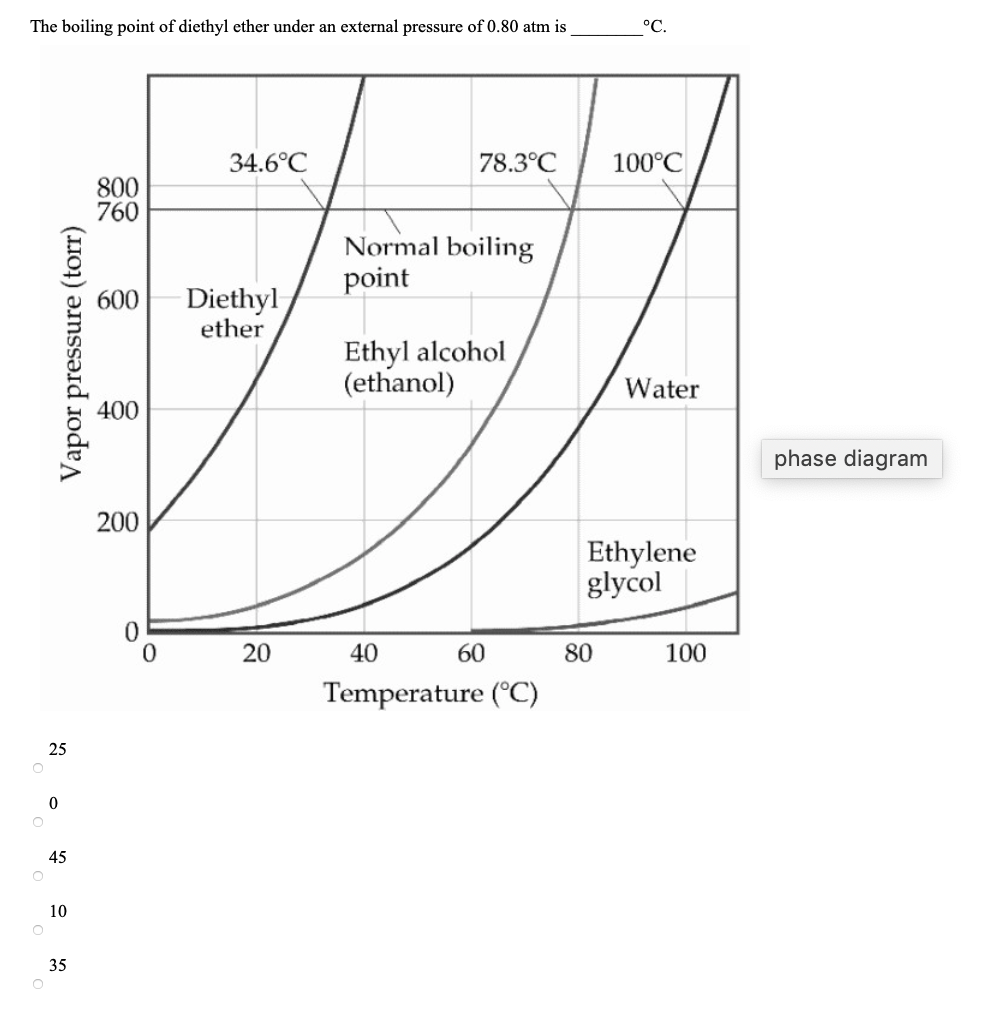
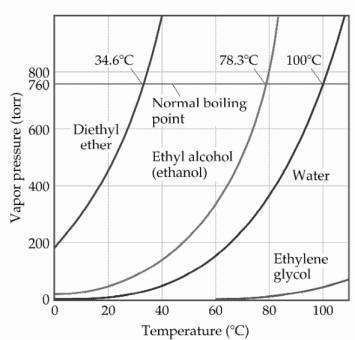
SOLVED: Based on the figure below, the boiling point of diethyl ether under an external pressure of 600 torr is 34.6°C. The normal boiling point of diethyl ether is 78.3°C. The boiling

The boiling point of diethyl ether CH_3CH_2OCH_2CH_3 is 34.50 degrees C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in diethyl ether is cholesterol . How many grams of cholesterol, C | Homework.Study.com

The boiling points of water, ethyl alcohol and diethyl ether are 100^∘C, 78.5^∘C and 34.6^∘C respectively. The intermolecular forces will be in the order of: