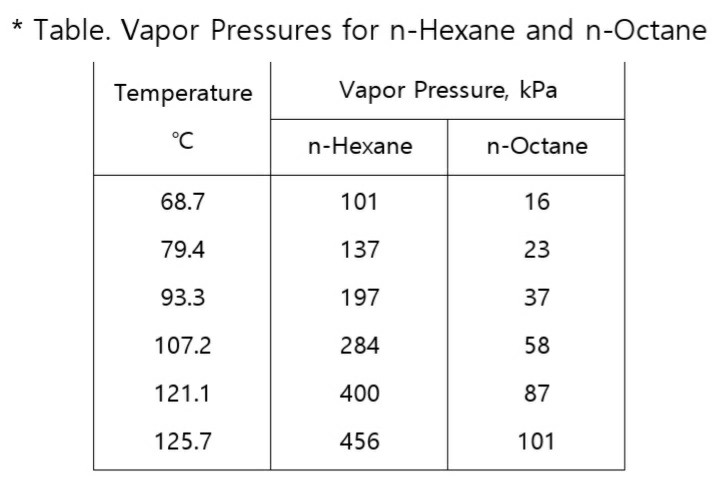
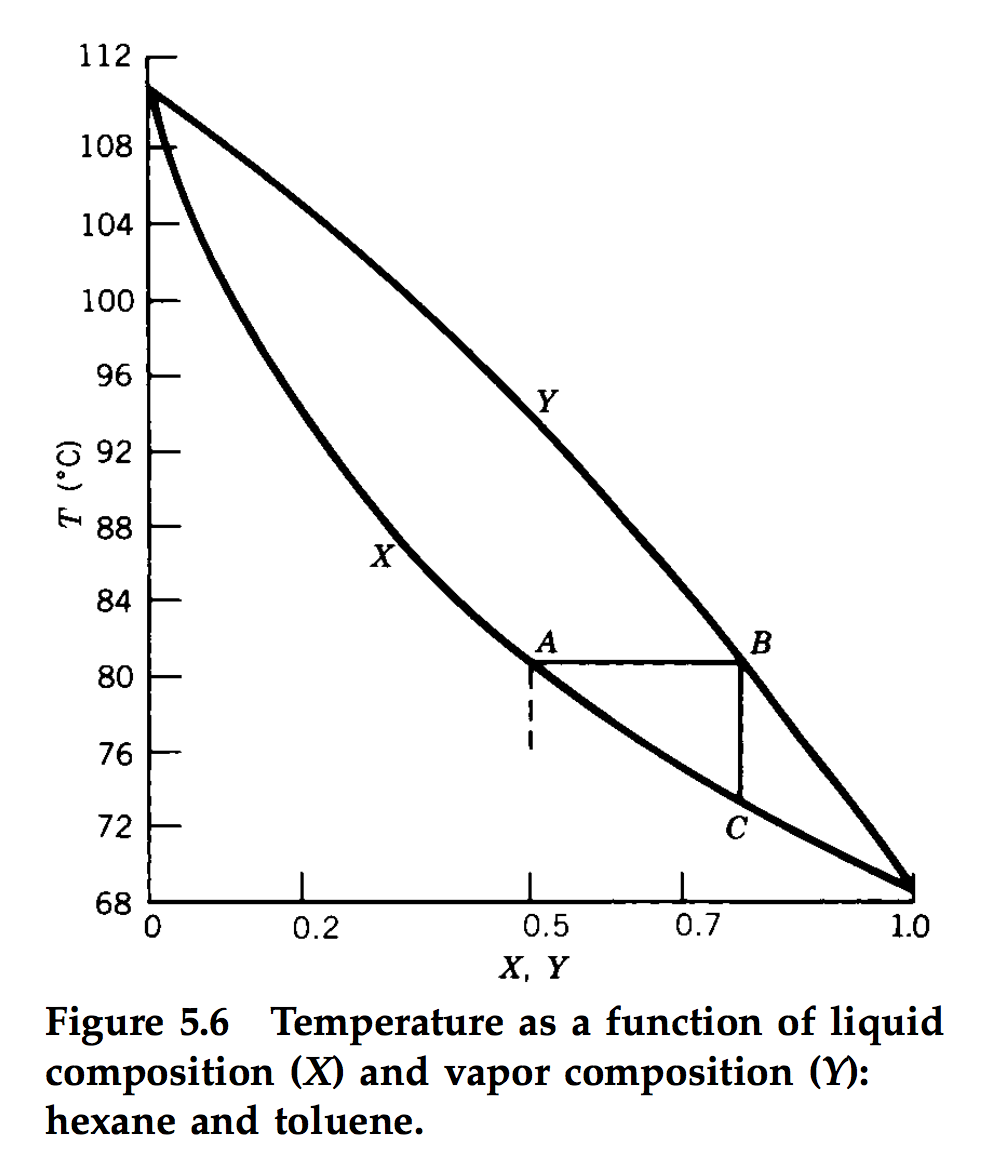
Boiling point of distillate as a function of weight fraction of hexane | Download Scientific Diagram

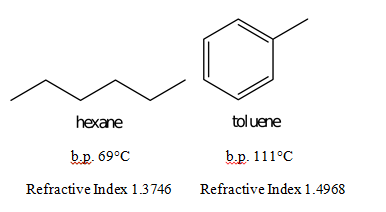
organic chemistry - Why do cyclic hydrocarbons have higher boiling points than their acyclic isomers? - Chemistry Stack Exchange

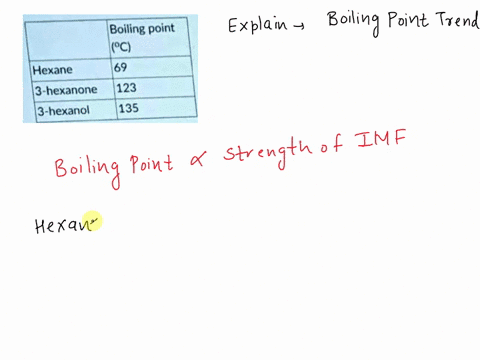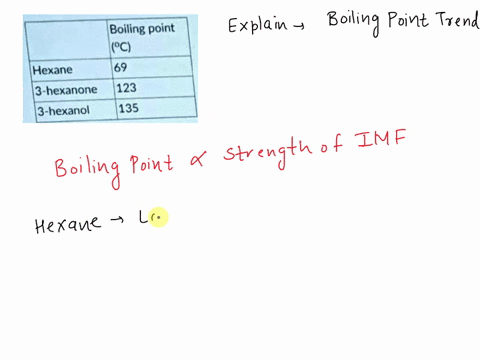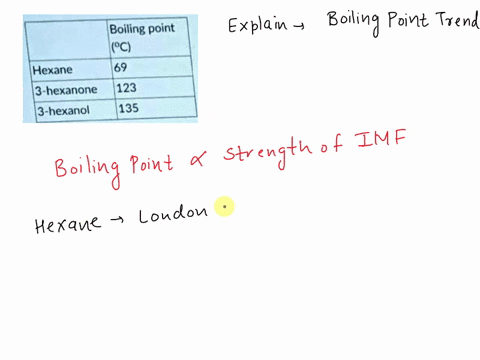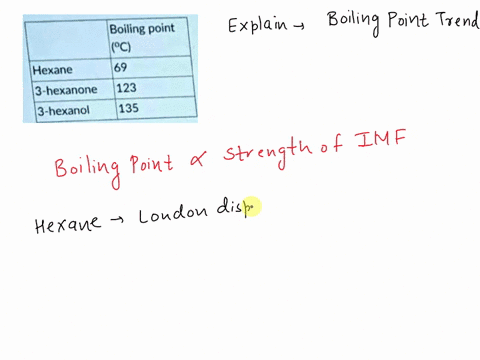
Explain which of the compounds hexane, 3-hexanon, and 1-hexanol is expected to have the highest boiling point and which one has the lowest boiling point. | Homework.Study.com
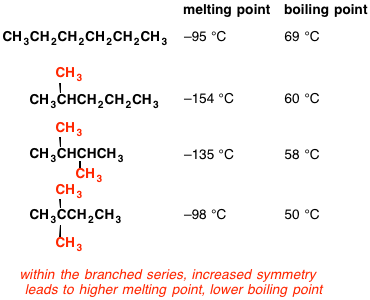
Why does hexane boil at 68°C whereas 2-methylpentane boils at 60.3°C yet the two compounds have the same molecular mass? - Quora

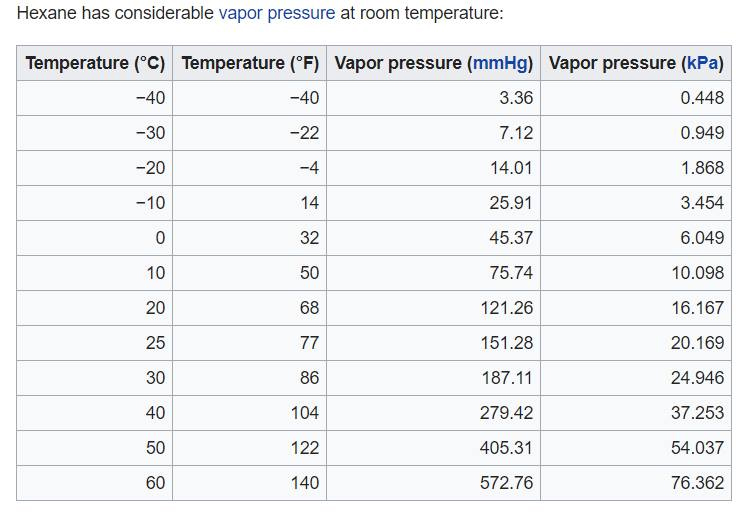
Evaporation (p, T ) diagram for liquid n-hexane. The solid line is for... | Download Scientific Diagram
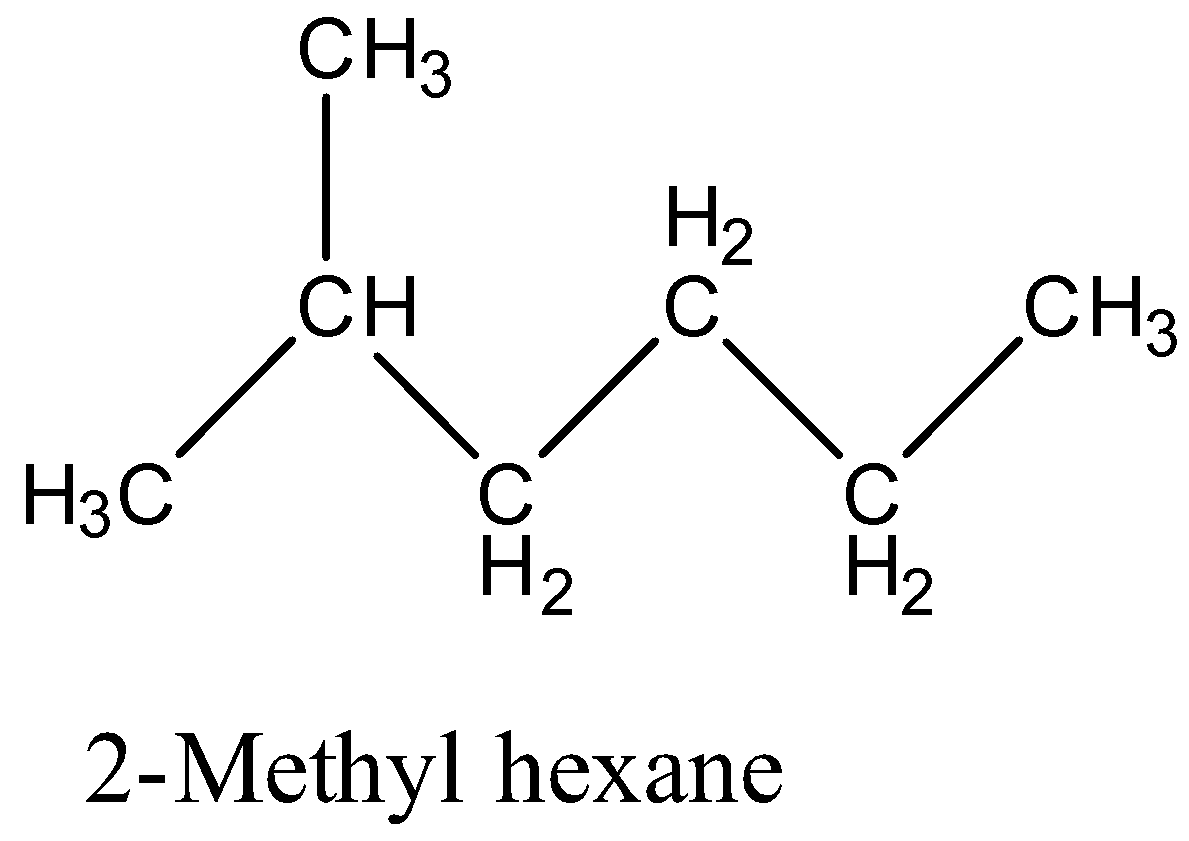
Between 2-Methyl hexane and 2, 2-Dimethyl pentane which has a higher boiling point?(a)- 2-Methyl hexane(b)- 2, 2-Dimethyl pentane(c)- Both have an equal boiling point(d)- None of these